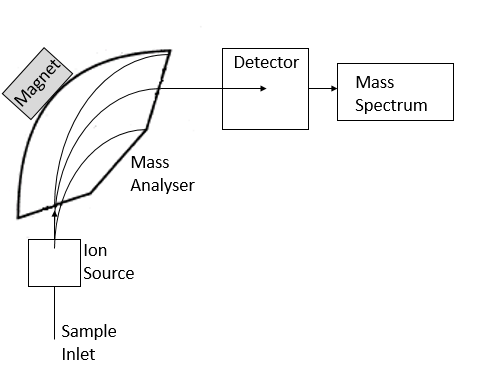
Mass Spectrometry
Ionisation
As the sample (referred to as the analyte) is introduced into the mass spectrometer (often after separation by chromatography), it is ionised in the ionisation chamber. It is necessary to ionise the sample so that the molecules can be manipulated and separated in order to identify them. The ionisation process refers to knocking one or more electrons from the analyte, producing a positively-charged ion (known as a cation). This is often achieved by a heated filament which emits electrons which can be accelerated and focussed to knock electrons off the sample molecules, thus producing ions. The ionisation method described refers to electron ionisation (EI), perhaps one of the most common ionisation methods utilised. However there is a wide range of alternative methods, including atmospheric pressure chemical ionisation (APCI), chemical ionisation (CI) and matrix-assisted laser desorption ionisation (MALDI).
Analyser
Once the analyte has been turned into this charged particle, it can be manipulated to enable detection. First the ion is accelerated (all ions produced from a sample will be accelerated so that they have the same kinetic energy). Next, a magnetic field can be used to deflect the ions, which will result in ions being separated depending on their mass. For instance, a lighter ion will be deflected with greater ease compared to a heavier ion, thus different components will take a different flight path. Similarly, the more charged an ion is (for instance if more than one electron was removed), the more it will be deflected. The combination of the mass of an ion and its charge gives the mass-to-charge ratio (m/z) of that particular ion. The strength of the magnetic field can be varied so that only ions with a specific mass-to-charge ratio are detected. As with ionisation, there is a variety of types of mass analyser available, including quadrupole, time-of-flight (TOF), and magnetic sectors.
Detection & Interpretation
The separated beams of ions then reach the detector, where the beam of ions will be directed into a metal box known as a faraday cup. When an ion hits this cup, an electric current is produced which is proportional to the number of ions hitting it. So the more of a particular chemical in a sample, the more ions are produced and so the greater the response. After detection, a mass spectrum will be produced for every individual component detected within the sample mixture. A mass spectrum appears as a type of graph, with the mass-to-charge ratio along the x-axis and the relative intensity or relative abundance along the y-axis. In short, it displays which molecules of specific mass and charge are present and in what relative amounts. It is using this spectrum that the analyst can determine what compound is present in their sample.
The largest peak in a mass spectrum is the base peak, which is usually given an arbitrary relative intensity of 100. All of the other peaks are measured in comparison to this peak. The peak observed at the highest m/z ratio will often be the molecular ion peak (M+), which relates to the molecular mass of the analyte. For example, hexane has a mass of 86. If this molecule was present in the sample, its mass spectrum would contain a molecular ion peak at m/z 86 (assuming the molecule obtained only a single charge following ionisation). However there would also be other lines present at other m/z values.
The other lines present in the spectrum are the result of the analyte breaking apart. As previously stated, in the ionisation chamber the sample is bombarded by electrons with the aim of knocking an electron off the analyte in order to produce an ion. However this beam of electrons sometimes has enough energy to not only remove an electron from the sample molecule, but also break it into smaller pieces. This is known as fragmentation. When fragmentation occurs, additional peaks will be seen in the mass spectrum that can be associated with the fragmentation of the molecule at different sites (although it is rarely possible to establish the origin of all fragment ions observed). It is these fragmentation ions that are vital in identifying compounds. Many different molecules share the same molecular weight and thus are likely to produce a molecular ion at the same m/z value, however in most cases the ion peaks caused by fragmentation will differ, allowing them to be distinguished from one another. Compounds can be identified based on their mass spectra with the use of mass spectral libraries and the analyst’s own expertise.
Applications
The possible applications of mass spectrometry techniques to forensic science are plentiful and, as technology advances, are becoming increasingly diverse and reliable.
The technique has proven particularly beneficial in the analysis of illicit substances, allowing investigators to identify unknown suspected drug samples and provide confirmatory evidence following presumptive drug tests (presumptive drug tests can only indicate the possible presence of a drug, but are not specific nor reliable enough to conclude). This may involve the analysis of actual suspected drugs, or tissue samples and bodily fluids can be subjected to such analyses to establish whether illicit drugs or toxic substances have been taken by an individual, knowingly or otherwise.
Analyses of samples pertaining to arson investigations are commonly subjected to gas chromatography-mass spectrometry due to the volatile nature of the samples. Suspected accelerants can be collected at the scene and analysed to confirm whether the substance in question is a flammable substance that may have been introduced to the scene maliciously. Similarly, mass spectrometry-based techniques are frequently used in the analysis of explosives or gunshot residues. Following the detonation of a bomb, little may remain of the original device other than unidentifiable debris. However analysis of such remains, in particular any traces or residues remaining on them, can indicate what type of explosive substance was used and potentially even the manufacturer, depending on the specific mix of components.
The analysis of trace evidence can be greatly benefitted by mass spectrometry, particularly when attempting to distinguish between or link different samples of apparently identical paint or dye. The specific chemicals used in a particular brand may be sufficient to establish whether two samples are likely to have originated from the same source. Likewise, mass spectrometry techniques have been utilised in distinguishing between different ink samples, potentially allowing investigators to establish the likelihood of two documents being written at the same time or by the same person based on the inks used in each document.
A technique known as isotope ratio mass spectrometry further extends the possible applications of these techniques, allowing for the study of specific elements and isotopes in a sample to aid in determining the age of a sample based on isotopic dating, establishing the likely origin of an individual by examining the ratios of different isotopes in their body, and distinguishing between seemingly identical substances through varying isotopic ratios. More can be read about this technique and its applications on the isotope ratio mass spectrometry page.
Unlike some analytical tests used in forensics, mass spectrometry can often definitively identify the components within a sample. Not only can the technique be used to identify samples (known as qualitative analysis), it also enables the analyst to quantify individual components. For example, gas chromatography-mass spectrometry is able to detect the presence of an illicit substance in an individual’s body, but also establish exactly how much is present. Such techniques are even becoming increasingly portable, opening up the possibility of in-situ analysis, particularly beneficial in airports or within the police force. Unfortunately mass spectrometry is a destructive technique, which is not ideal in forensic investigations if there is a limited amount of sample available for analysis.
University of Leeds. An Introduction to Mass Spectrometry. [online] Available:http://www.astbury.leeds.ac.uk/facil/MStut/mstutorial.htm
Michigan State University. Mass Spectrometry. [online] Available: http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/Spectrpy/MassSpec/masspec1.htm
University of Arizona. Introduction to Mass Spectrometry. [online] Available: http://www.chem.arizona.edu/massspec/intro_html/intro.html